
Tuesday, February 3, 2009
Sunday, February 1, 2009
Iron Deficiency Anemia (IDA)
 Anisocytosis with hypochromia and microcytes (IDA)
Anisocytosis with hypochromia and microcytes (IDA)Iron-deficiency anemia (IDA) is the most commonly encountered anemia and may be due to [1] impaired iron intake, [2] pregnancy, [3] intravascular hemolysis, [4] hemorrhage, or [5] lactation. IDA most often affects women in their reproductive years and growing children. Each mL of packed RBC’s contain about 1.0 mg of iron. The average adult contains about 3.5 to 5.0 grams of iron. They will ingest about 15 to 20 mg of iron daily, excreting most of it. The body normally absorbs about 1 mg of iron (which is equal to the daily loss).
The iron taken into the body is in the ferric state (Fe+3) in the stomach, it is changed to the ferrous state (Fe+2). Ferrous iron is absorbed by the small intestine and in the intestinal capillary system, iron is bound to transferrin to form a protein-iron complex. This complex is carried to the bone marrow (and other cells requiring iron) and will bind to the cell. The complex is absorbed into the cell, the iron released, and the transferrin moves back into the blood stream. Inside the cell, the iron is bound to the protein apoferritin to form ferritin. The ferritin combines to form aggregates which forms brown pigment granules called hemosiderin. IDA is a hypoproliferative, microcytic, hypochromic anemia due to ineffective RBC and/or hemoglobin production.
If IDA is observed in a healthy appearing adult male, the physician should look for a gastrointestinal lesion that may be losing blood. The menstruating woman will lose between 50 and 70 mLs of blood monthly and when the iron is not being replace anemia will result. It is estimated that up to 20% of the women in the US have IDA. IDA produces a hypochromic, microcytic anemia. On the peripheral blood smear, the erythrocytes are hypochromic and microcytic. If IDA is severe, poikilo-cytosis and anisocytosis may be obvious. In the bone marrow, the rubriblasts will be poorly hemoglobinized and demonstrate ragged appearing cytoplasm. The serum iron will be decreases and the total iron binding capacity (TIBC) will be increased. The ferritin level will be less than 20 ng/mL.
IRON TRIVIA
If dietary iron is from meat sources, it is heme bound. Vitamin C is not required for absorption. If this iron is from eggs and vegetables, it is in the ferritin and hemochrome bound form and requires vitamin C for optimum absorption. In the stomach, the gastric fluid and pepsin releases iron which passes into the gastrointestinal tract. Most iron absorption occurs in the duodenum. Some iron absorption will occur in the jejunum and ileum.
In developed countries, adequate ion intake is not a problem. The high risk groups who are most likely to develop IDA are [1] infants, [2] rapidly growing adolescents, [3] pregnant women, and [4] women during their child bearing years (losing from 10 to 45 mg/month). The pregnant female need about 3.4 mg of iron daily (a total of 1000 mg to carry the fetus to term). About 400 mg are needed for the fetal RBC mass. At parturition, approximately 300 mg will be lost and up to 170 mg will be contained in the placenta and umbilical cord.
It has been estimated that a healthy adult male would require about eight years to develop IDA if no more iron were absorbed in his diet. Malabsorption is uncommon unless there is a primary problem as [1] sprue, [2] gastrectomy, or [3] atrophic gastritis. Other causes are [1] regular blood donations and [2] paroxysmal nocturnal hemoglobinuria.
CLINICAL SYMPTOMS
Early stages (stage 1) are generally asymptomatic. As IDA develops into stage two, the depletion of the body’s iron stores occurs, with the patient experiencing hypoxia, characterized by lethargy and asthenia. As stage two progresses, iron deficiency is demonstrated by a decrease in erythropoiesis as iron is no longer being inserted into the hemoglobin molecule. Lab testing will show decreases in serum iron, increased total iron binding capacity (TIBC), and low transferrin saturation.
As the IDA progresses into the stage three level, the mitotic activity of the RBC increases resulting in small erythrocytes (microcytes) and hypochromia. When the hypochromic microcyte is observed in the blood film, there is also anisocytosis and poikilocytosis and IDA if fully expressed. Symptoms that begin to appear in stage two and are fully expressed in stage three are [1] ankle edema, [2] exertional dyspnea, [3] headaches, [4] glossitis, [5] koilonychia, [6] pallor, [7] pica, and [8] tachycardia. In the woman of child bearing age, [1] menorrhagia, [2] irregular cycles, and/or [3] amenorrhea may occur.
CLINICAL LABORATORY FINDINGS
RBC count: Usually normal at the beginning. The count will usually remain within normal limits unless the iron stores are severely depleted.Hemoglobin: Will undergo greater degrees of reduction.
Individual are to be considered anemic if hemoglobin values fall as indicated in g/dL:
- [1] Children (from 6 months to 5 years) less than 11
- 2] Children (from 6 years to 14 years) less than 12
- Adult men. . . . . . . . . . . . . . . . . . . . . . less than 13
- Adult women. . . . . . . . . . . . . . . . . . . less than 12
- Pregnant women . . . . . . . . . . . . . . . . less than 11
RBC Indices: Anemia is suspected when the values fall as indicated:
- [1] MCV . . . . . 75 to 80 fL
- [2] MCH . . . . . 25 to 27 pG
- MCHC. . . . less than 32 percent
- [4] If the MCV is less than 75 fl.
- If the MCH is less than 25 pG
Retic Count: This test parameter may be normal or decreased in early IDA. As the IDA progresses, the retic count decreases
Fragility: This test will usually be normal. If codocytes (target cells) are present, then one may see a decreased value. The value of this test is in detecting hereditary spherocytosis
WBC: The count is usually normal as is the differential
Platelet count: This testing parameter is usually normal
Serum Iron: decreases in stages as IDA develops
Normal values (μg/dL) as follows
- Newborn . . . . . . . 100 to 250
- Infant . . . . . . . . . 40 to 100
- Child . . . . . . . . . 50 to 120
- Adult male. . . . . . 65 to 170
- Adult female . . . . 50 to 170
Serum Ferritin: Is an indicator of how much iron is being stored and it will progressively decrease as IDA develops. It is the major iron storage compound and is found in all body cells. It is a protein that is complexed with iron. If iron is absent from this protein, it is then know as apoferritin. This is an important test in differentiating IDA from other types of microcytic normocytic anemias as it will be increased in thalassemia and sideroblastic type anemias. Normal values for men are 15 to 200 μg/L and women are 12 to 150 μg/L
Generalized test findings as IDA develops:
[1] In the initial stages when patient is asymptomatic:
- A. Serum ferritin will be decreased
- B. Bone marrow iron will be decreased
[2] In the second stage, when erythropoiesis is occurring withoutiron to insert in the heme portion of the hemoglobin molecule:
- A. Serum Ferritin continues to be decreased
- B. Bone marrow iron continues to be decreased
- C. Serum iron is now decreased
- D. TIBC is increased
[3] In the final stage with fully developed IDA
- A. Serum Ferritin, bone marrow iron, and serum iron are decreased
- B. TIBC is increased
- C. Hemoglobin and hematocrit are decreased
- D. MCV is decreased
- E. RDW is increased
Blood Type

Hereditary Spherocytosis (HS)

The RBC membrane in this disorder is defective. Hereditary spherocytosis (HS) is characterized by some degree of spectrin deficiency in the cell membrane. The degree of the deficiency determines the severity of the disorder. The RBC membrane consists of “skeletal’ proteins: [a] spectrin (comprising the major cytoskeletal component), [b] actin, [c] protein 4.1, [d] protein 4.9, and [d] ankyrin. There are ten other major proteins and several minor proteins. There is also a decrease in the membrane lipids in HS. The erythrocyte with abnormal cytoskeletal proteins are unstable and have less flexibility.
This means that they are more easily trapped in the spleen because it takes them longer to make the transit through this organ. The membrane defects also affect membrane permeability to Na+ and K+. The impaired membrane with its permeability defects will allow the accumulation of Na+ into the cell and the escape of K+. The accumulation of intracellular Na+ causes the cell to swell with water forming a hydrocyte. These cells have the appearance stomatocytes which are easily removed and destroyed in the spleen. If the K+ loss is significant, then there is cell shrinkage and the cell is referred to as a xerocyte with the same fate as the hydrocyte. If Ca++ accumulates, then the cell becomes an echinocyte in its appearance.
The echinocyte is reported to become a spherocyte when it transverses the spleen. If the defect in spectrin is limited to about 10 to 25% of the total spectrin, then only a mild anemia presents with small number of spherocytes. If the defect affects 25% to 50% of the total spectrin, then the number of circulating spherocytes is from 2+ to 3+ with a moderate anemia expressed. If the defect affects more than 50% of the total spectrin, then both spherocytosis and anemia is severe. Refer to the following illustration. The three blocked in areas identify different areas of defect.
Clinical features of hereditary spherocytosis includes [a] splenomegaly, seen in >50% of cases and [b] jaundice. Other rare symptoms include [a] spinal cord dysfunction, [b] myocardiopathy, and [c] leg ulcers.
Complications include:
....Viral infections, of which most are mild, can precipitate a hemolytic crisis. Serious infections can initiate an aplastic crisis which may lead to heart failure and the requirement of transfusion therapy. It has been found that an infection with parovirus results in viral invasion of the hemopoietic stem cells, inhibiting their growth.
....A predisposition toward the bilirubin-type gallstones. All ages are vulnerable with the greatest frequency occurring at 50 y/o.
....Gout.
....Chronic erythematosus condition (a redness of the skin due to capillary dilation).
Expected clinical laboratory findings include:
....Anemia varying from mild to severe
....Hemoglobin will be normal or decreased
....Retic count: usually > 8.0%
....Presence of spherocytes (the most reliable finding)
....MCV = 77- 87 fL (low)
....MCH = 27 - 31 pG (normal)
....MCHC = >36% in 50% of the cases
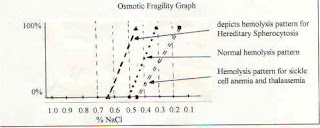
....Osmotic fragility test is increased
....Bilirubin = >1.5 mg/dL
....LDH = > 200 IU/L
....AHG = negative
SPLEEN FACTS
The spleen consists of white pulp, a network of lymph or lymph-like nodes with leukocytes consisting predominately of lymphocytes. The red pulp is a network of reticular fibers filled with blood sinuses. The blood sinuses are separated with “cords” of lymph tissues that contain macrophages and other cells. Narrow elliptical fenestrations separate the “cords” from sinuses. The splenic environment is hypoxic, acidic, and hypoglycemic. The normal RBC will transit the splenic environment in 30 seconds. Damaged and abnormal RBC’s are detained from minutes to hours in this hostile environment that is a taxing stress to less than normal RBC’s.
When the spherocyte transverses this environment, it is destroyed for the following reasons.
....Spheroid shape
....Cytoplasmic viscosity of the RBC which enhances intracellular dilution and RBC swelling.
....Membrane permeability
....Inability to maintain the ATP membrane pump because of the loss of glycolysis
Detrmination of Glucose-6-Phosphate Dehydrogenase in Erythrocytes
Glucose-6-Phosphate + NADP+ <=> 6-Phosphogluconolactone + NADPH
Individuals whose G6PDH is partly inactive are prone to oxidative stress. This arises because NADPH produced by the pentose phosphate pathway is required for reduction of glutathione, which, in turn, helps reduce peroxides. In the absence of sufficient NADPH, glutathione remains oxidized and peroxides cannot be neutralized.
In this of experiment we have carried out determination of glucose-6-phosphate dehydrogenase in erythrocyte which is a significant enzyme in pentose phosphate pathway. When G6PD is present in erythrocytes at normal level, it can act on glucose-6-phosphate and catalyses NADP+ to NADPH which in turn will emit UV light, absence of UV light indicates a decrease in the enzyme level. A decreased level of G6PD enzyme in erythrocytes will cause reduction of NADPH production and consequently hemolysis will occur (hemolytic anemia).
A mutation present in some populations causes a deficiency in glucose-6-phosphate dehydrogenase with consequent impairment in the generation of NADPH. This impairment is manifested as red cell hemolysis when the susceptible individual is subject to oxidants such as the antimalarial primaquine, aspirin, or sulfonamides or when the susceptible individual eats fava beans (Vicia fava).
The Pentose phosphate pathway supplies RBC with NADPH to maintain the reduced state of glutathione. The inability to maintain reduced glutathione in RBC’s leads to increased accumulation of peroxides, predominantly H202 that in turn results in a weakening of the cell wall and concomitant hemolysis.Therefore, any defect in the production of NADHP could, therefore, have profound effects on erythrocyte survival.
Hemolytic anemia is the red blood cell (RBC) plasma membranes rupture prematurely. The released haemoglobin pours into the plasma and may damage the filtering unit (glomeruli) in the kidneys. The condition may result from inherited defects such as abnormal red blood cell enzymes, or from outside agents such as parasites, toxins, or antibodies from incompatible transfused blood.
The symptom of hemolytic anemia is the reduced number of RBC’s or a decreased amount of haemoglobin in the blood. The person feels fatigued and is tolerant of cold, both of which are related to lack of oxygen needed for ATP and heat production. Also, the skin appears pale due to the low content of red-colored haemoglobin circulating in skin blood vessel. That is why G6PD determination or verification is very significant especially for new born babies as a screening test.











































































































